Understanding Sintering in Metal Clay
All metal clays are made of microscopically powdered metal, an organic binder and water. The binder holds enough water to make metal clay malleable, which allows it to be handled as a soft clay. After a metal clay design is completed, it is then fired at temperatures that are specific to the clay type produced by each individual manufacturer. During the firing process, excess water evaporates, binder is incinerated, and the metal particles move closer together to form a solid mass.
The Better the Molecular Bond, the Stronger the Piece
The removal of the binder and water creates spaces between the metal particles as they move towards and bond with each other. The longer a piece is fired at the correct temperature, the closer these bonds become, resulting in stronger pieces. However, because firing temperatures are lower than those that would cause a full melt, the resulting metal mass will always be somewhat porous.
A simplified form of powder metallurgy, this method of bonding individual metal particles with heat is known as sintering. Sintering has been in use for thousands of years, most commonly from the beginning of the twentieth century. Its use in jewelry-making began when metal clay was developed in the early 1990’s.
Picturing the Bonding Process
An analogy developed by Hadar Jacobson, creator of Hadar’s Clays, may help to explain the process better. Start by imagining a bowl of ice cubes in the freezer. Ice melts at 32°F/0°C. The temperature in the freezer is far below that. What happens if we raise this temperature without reaching the melting point? The ice cubes will start sticking to each other until we are able to pick them up as one solid unit. However, since they don’t touch each other at every point of their surface, there are spaces between them and this whole mass is porous. When the ice is brought above its melting point it becomes liquid and flows to fill all remaining spaces or pores.
The illustration below provides a more visual example.
- Dry metal clay: Imagine the first image is dry metal clay. You can see that the metal particles (depicted in gray) are only touching with small connection points. This mass looks and feels solid, but can be easily broken.
- Torch fired or kiln firings less than manufacturer’s recommendations: The middle image might reflect the quality of the bond when metal clay is torch fired, kiln fired at low temperatures, or kiln fired at high temperatures – but for a short time. The bonds are much better and the piece is not as fragile, but it is not quite as solid as it could be.
- Optimal firing: The third image shows what happens when metal clay is fired at its optimum time and temperature. It’s much more solid with smaller and fewer spaces.
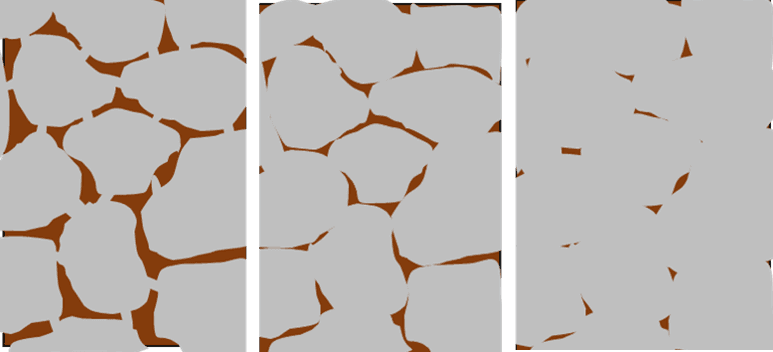
L to R: Dry metal clay, torch fired or kiln fired less than optimally, optimally fired clay.
Oxidation and Molecular Bonding
Pure metals such as fine silver and gold will sinter very well in what is referred to as an open shelf kiln environment, where the clay is exposed to the oxygen in the atmosphere as it fires. The presence of oxygen also ensures that the binder can be fully burned off, allowing the microscopic particles of metal the best chance of bonding completely.
Two-Phase Firing Prevents Oxidation Of Alloys And Base Metal Clays
Clays such as .925 sterling, bronze, steel, and all other base clay variations are prone to oxidation, which prevents the metal particles from bonding. Instead, these alloys must be fired surrounded by granulated carbon, which reduces the amount of oxygen in the kiln and facilitates sintering.
Because the flame of a torch consumes most of the oxygen in its immediate area, small pieces of pure copper and some bronzes can be torch fired (while exposed to air for a very short time) before they start to oxidize internally. However when they are, the crust of surface oxidation that forms may flake off, taking with it delicate texture and details. Steel cannot be torch fired for any length of time.
Therefore, base metals are most commonly fired in a stainless steel or ceramic fiber container buried in carbon, which reduces the amount of oxygen and inhibits oxidation. Because it is important that the binder burns off completely before sintering starts, a two-phase process ensures a successful outcome:
- The first phase is fired in air so the binder can burn out, and
- the second phase is fired in carbon so the particles can bond together.
This is achieved by making sure that the ramping time (the time it takes for the kiln to reach the desired sintering temperature) is at least 45-60 minutes. The hold time after ramping may differ from manufacturer to manufacturer and from exact clay body to clay body. With differences between every kiln manufactured (even those manufactured by the same company), a test firing is always advisable.

Steel container and carbon for reduced oxygen kiln firing

Torch firing small copper or bronze pieces works if the instructions state they can be fired this way.
Shrinkage
Another result of the sintering process is shrinkage. As it transitions from fresh, malleable clay to hard, dry greenware, some of the water content evaporates and metal clay will shrink about 1%. When the binder and all remaining moisture are removed during the firing process, the metal particles move closer together, and another percentage of the mass is eliminated, so the piece shrinks more (how much depends on the exact clay type). Metal clay shrinks in all ways – height, width, length, circumference and thickness.
Allowing for shrinkage effects when firing
Because many factors can affect the movement of the clay as it shrinks, proper support of the piece and its positioning in the kiln or firing container need to be carefully considered.
Open Shelf Firing
- Anything that interferes with the movement of the clay as it shrinks can cause warping or uneven shrinkage.
- Insufficient support of a piece can cause slumping.
- For hollow forms, support materials such as fiber blanket or vermiculite can be used.
Carbon Firing
- When firing rings or hollow forms in carbon, care must be taken to ensure the carbon won’t fill the holes or voids completely and interfere with the shrinkage required.
- Make a stainless steel mesh cage to cover the work
- OR block the void with a thin piece of fiber blanket or fiber paper
Shrinkage is also affected by the condition of the clay. Fresh clay, right out of the package, may shrink differently than clay that has been previously worked with and rehydrated.
Fully understanding sintering, shrinkage and optimal firing schedules will greatly improve successful use and firing of all metal clays.
Pam East has written an article comparing sintered silver with fabricated silver. It’s a great resource if you are about to make a decision between making something from metal clay or silver sheet/wire. It will help you evaluate the qualities your project needs and what is best to use. Click here.